World Thrombosis Day 13 October 2021: Reduce APS Knowledge Gap
Antiphospholipid syndrome (APS) is linked to one-third of new strokes occurring in individuals younger than 50 years, and may be responsible for up to 1% of all thrombosis. APS is a life-threatening systemic autoimmune disease that causes excessive blood clotting. Immune system mistakenly produces antibodies (antiphospholipid antibodies [aPL]) against certain normal proteins in the blood. These antibodies can cause potentially fatal events such as strokes, heart attacks, and blood clots in the legs (deep vein thrombosis) and lungs (pulmonary embolism). Antiphospholipid Syndrome is the most important treatable cause of recurrent miscarriage; it is also associated with other pregnancy complications such as pre-eclampsia, low weight babies, and premature births. Despite increased recognition of APS, much remains to be learned about the cause, full range of symptoms, diagnosis, and treatment for APS. APS can be detected by antiphospholipid (aPL) antibodies, including lupus anticoagulant (LA), anticardiolipin (aCL) antibody, and/or anti-β2 glycoprotein I (aβ2 GPI) antibody assays. APL antibodies can assist the determination of the risk of thrombotic and obstetric events, and consequently the intensity of treatment.
D-dimer level is another independent predictor of recurrence in patients with an unprovoked VTE. The combination of aPL and D-dimer detection can serve as early diagnosis, severity classification, therapy monitoring, and discharge assessment.
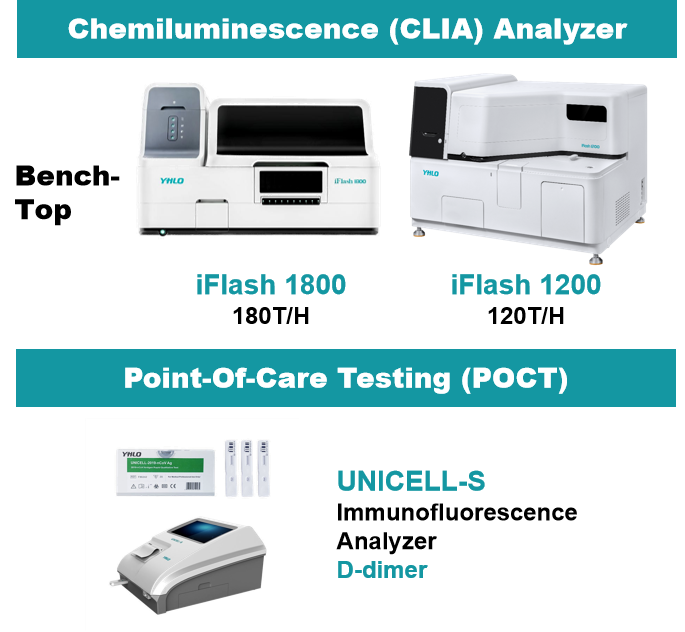
YHLO provides one-stop APS screening for early diagnosis and timely treatment to prevent future problems.

Attachments:
MORE NEWS
n November 18, 2025, YHLO’s 2025 Global Core Distributors Conference was successfully held in Düsseldorf, Germany. Under the theme “Academia-Driven, Shared Value,” the meeting brought together representatives from more than 30 core distributors worldwide. YHLO’s founder, Mr. Deming Hu, and Chairman, Mr. Kunhui Hu, led the company’s executive team to attend and discuss strategic development with partners.
2025-11-20
YHLO Secures Approval for the World’s First sCD146 CLIA Assay
YHLO has obtained approval from the Guangdong Medical Products Administration for its soluble CD146 (sCD146) CLIA assay, marking a major milestone in central nervous system (CNS) disease diagnostics. YHLO is now the first company worldwide to offer an sCD146 test for cerebrospinal fluid, bringing its total CLIA assay portfolio in China to 173.
2025-11-18
Add: Building 1, YHLO Biopark, Baolong 2nd Road, Baolong Subdistrict, Longgang District, 518116 Shenzhen, P.R.China







