YHLO COVID-19 Rapid Ag Test Kits(self-test) Approved by BfArM
Summary:
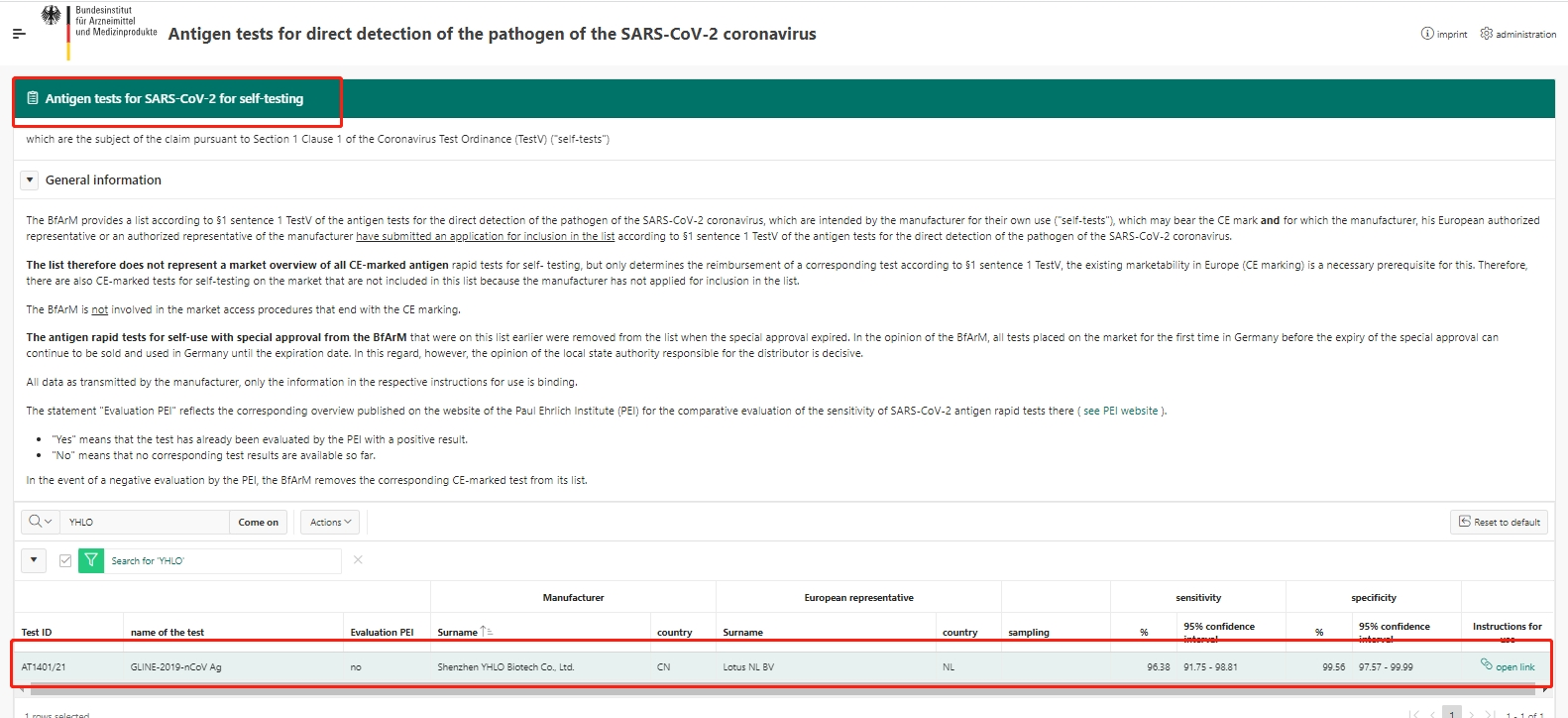
YHLO COVID-19 self-test kit, GLINE-2019-nCoV Ag assay was granted special approval by the German Federal Institute for Drugs and Medical Devices (BfArM) in Germany.
Early besides from Germany, the performance of YHLO’s GLINE-2019-nCoV Ag assay has already been validated by different governments, such as ANSM from France, DHSC from the UK, MOH from Italy, SEI from Spain, etc. In December 2021, YHLO’s COVID-19 self-test kit also received the EC Certificate, which means this product is well-qualified to be applied in the European market.
GLINE-2019-nCoV Ag assay is a colloidal gold immunoassay for the rapid qualitative determination of nucleocapsid protein of SARS-CoV-2 in human nasal or nasopharyngeal swab. The rapid antigen test is easy to operate compared with PCR testing. By following simple instructions, the results would be obtained in 15 minutes without any electronic equipment.
YHLO always strives to provide accurate and efficient immunoassays to better help the world to fight against COVID-19.


+86 755 26601910
marketing@szyhlo.com
© 2020 Shenzhen YHLO Biotech Co., Ltd. All rights reserved. 粤ICP备17105123号






 Language
Language 




